Want to learn more about NeuroShield?
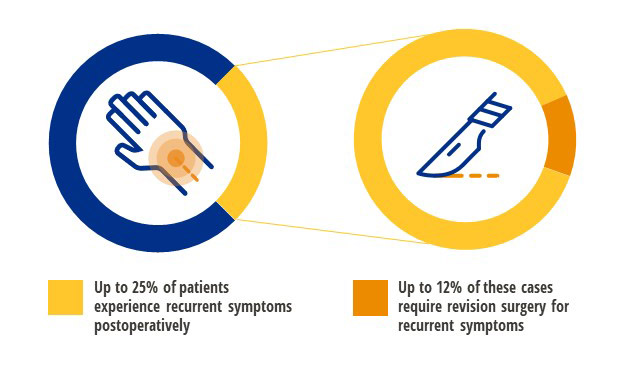
Contact usUp to 25% of patients experience refractory symptions after carpal tunnel surgery
Carpal tunnel release is one of the most commonly performed upper extremity procedures. The majority of patients experience significant improvement or resolution of their symptoms; however, a subset of patients will experience ongoing or recurring symptoms. Refractory symptoms of carpal tunnel can persist or reoccur after carpal tunnel release surgery in 1% to 25% of patients, with up to 12% of patients requiring secondary surgery.1-3
The most common cause of recurrent symptoms is scar tissue formation
The most common cause of recurrent symptoms returning after a period of symptom relief is due to scar tissue formation in the carpal tunnel postoperatively, leading to tethering of the nerve and recurrent compression, and in some cases reformation of a pseudo transverse carpal ligament.4-8
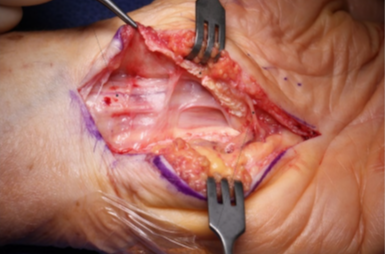
A scarred median nerve adherent to the undersurface of the radial leaf of the transverse carpal ligament in a case of a revision open carpal tunnel release. Proximal nerve bruising and swelling confirms ongoing compression despite the previous release (left hand, proximal to the left).
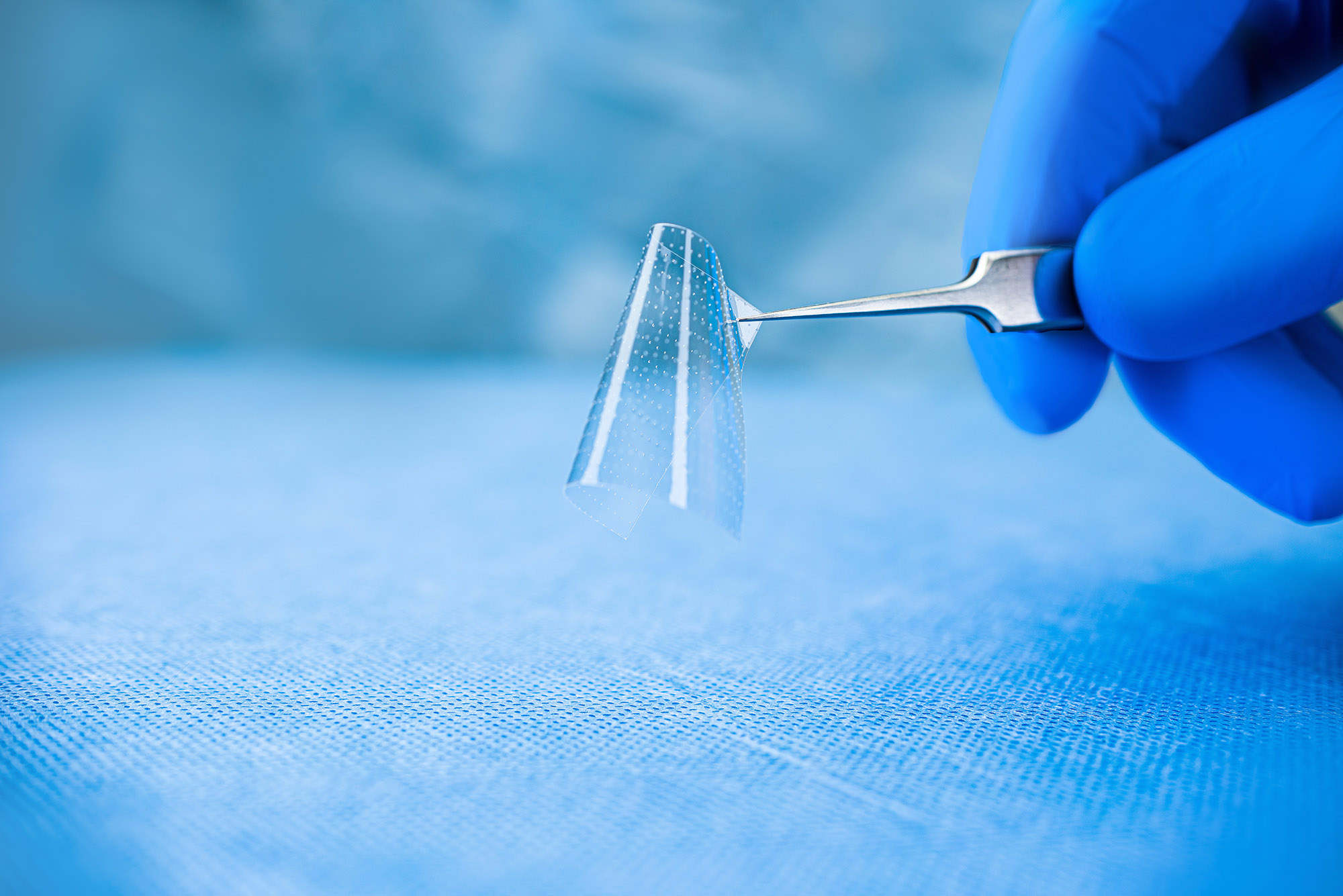
Reduce recurrent scar tissue formation with NeuroShield
Indicated for the repair of peripheral nerve injuries by providing a protective barrier during tissue healing, NeuroShield is comprised of chitosan with a low degree of acetylation. Preclinical, in vitro studies have shown that chitosan with a low degree of acetylation inhibits fibroblast migration and proliferation, while simultaneously supporting Schwann cell migration and proliferation.9-13
See the case report highlighting the use of NeuroShield in an open revision carpal tunnel surgery.
4030-MKT-039-A